
Key points
- Beam Therapeutics is a pioneer in its proprietary gene editing technique called base editing.
- Base editing can be more precise than CRISPR/Cas9 by editing just a single letter in the genome rather than cutting double strands to cut out specific sequences.
- Beam Therapeutics surprised investors, beating EPS by $2.42 to $1.73 versus a loss of 69 cents, while revenue rose 1,481% to $316.2 million versus $34.12 million , mainly because of the deal with Eli Lilly.
- 5 stocks we like better than Beam Therapeutics
Fascio Therapeutics Inc. NASDAQ: BEAM is a biotechnology company specializing in the development of precision gene therapies for inherited diseases. The medical company is pioneering a proprietary gene editing technique called base editing. Unlike CRISPR/Cas-9, popularized by CRISPR Therapeutics AG NASDAQ: CRSPmolecular scissors cut out specific sequences of the human genome, base editing allows modifications of single bases (letters) without causing double-strand breaks and risking unintended consequences of mutations or off-target effects.
Beam benefits from great partners
The company is a partner of Eli Lilly & Co. NYSE: LLYVerve Therapeutics as well Pfizer Inc. NYSE:PFE AND Apellis Pharmaceuticals Inc. NASDAQ: APLS on various projects. Beam’s recent Q4 2023 earnings report was similar to that of CRISPR Therapeutics, with positive EPS and revenue growth multiple. However, just like CRISPR Therapeutics, the majority of revenue was made up of licensing fees rather than sales. Check the heat map of the sector on MarketBeat.
The company reported a surprising earnings windfall
On February 27, 2024, Beam Therapeutics reported fourth-quarter 2023 earnings per share of $1.73 versus consensus analyst estimates for a loss of 69 cents. This was a beat to EPS of $2.42. Revenue increased 1,481% year-over-year to $316.2 million versus consensus estimates of $34.16 million. Revenue consisted primarily of Eli Lilly’s purchase of the rights to a group of gene-editing therapies developed in collaboration with Verve Therapeutics. This transaction included revenue of $214.4 million. The rest of the revenue came from payments from partners Apellis and Pfizer. This increase in revenue reduced Beam’s 2023 net loss by more than half to $132.5 million.
Beam is building a walkway for cash
The company still has a war chest of $1.2 billion in cash and cash equivalents. This gives the company a liquidity pathway through 2027. This includes foundational funding for BEAM-101, BEAM-301, BEAM-302 and ESCAPE therapies and continued investment in its manufacturing capabilities and platform advancements.
The company provided an update on its 2024 milestones
Beam provided an overview of the key expected milestones it hopes to achieve in 2024 per franchise.
For the sickle cell disease (SCD) franchise., Beam expects to complete dosing of patients in the sentinel cohort and begin dosing of patients in the expansion cohort in the first half of 2024 for the BEACON Phase 1/2 clinical trials for BEAM-101. The editing approach seeks to reactivate fetal hemoglobin. Beam will report initial data on more patients for the study in the second half of 2024. CRISPR Therapeutics and biography of the bluebird NASDAQ: BLUE received FDA approval on December 8, 2023 for their respective gene therapies for SCD.
It also hopes to initiate preclinical studies enabling Phase 1 as it continues to invest in its ESCAPE (Engineered Stem Cell Antibody Paired Evasion) conditioning platform.
For the franchise on genetic diseases, Beam has filed the European clinical trial application for BEAM-302 for the treatment of alpha-1 antitrypsin deficiency (AATD). AATD is a genetic disease that affects the lungs and liver. AAT is produced in the liver and is essential, as it helps protect against lung damage caused by protease enzymes. Patients with AATD produce deficient AAT, which impairs their protective function and can lead to chronic obstructive pulmonary disease (COPD) and emphysema.
Following acceptance by CTA, the BEAM-302 Phase 1 clinical trial is expected to begin in the first half of 2024. The company also plans to file an Investigational New Drug (IND) application for BEAM-301 for the treatment of glycogen storage disease type 1a (GSD1a). in the first half of 2024.
What did Beam’s CEO have to say?
Beam Therapeutics CEO John Evans commented: “This year has the potential to be transformative for Beam as we work to advance multiple foundational editing programs into the clinic, anchoring key high-value franchises through near-term catalysts, all supported by a solid balance sheet.”
Evan continued, “In 2024, we plan to initiate our first in vivo clinical studies and report the first human data from our ex vivo base modification clinical programs.”
JP Morgan has re-rated BEAM stock
On January 29, 2024, JP Morgan noted that the AATD treatment candidate has the potential to represent a $12 billion commercial opportunity as JP Morgan expects an early biomarker readout from its Phase 1 study. Analysts believe that each adjusted 10% incremental probability of success is worth about $14 per share. JP Morgan upgraded BEAM shares to Overweight with a $40 price target.
Beam Therapeutics Analyst Ratings and Price Targets I’m on MarketBeat. Beam Therapeutics peers and competitor actions can be found with MarketBeat Stock Screener.
 \
\
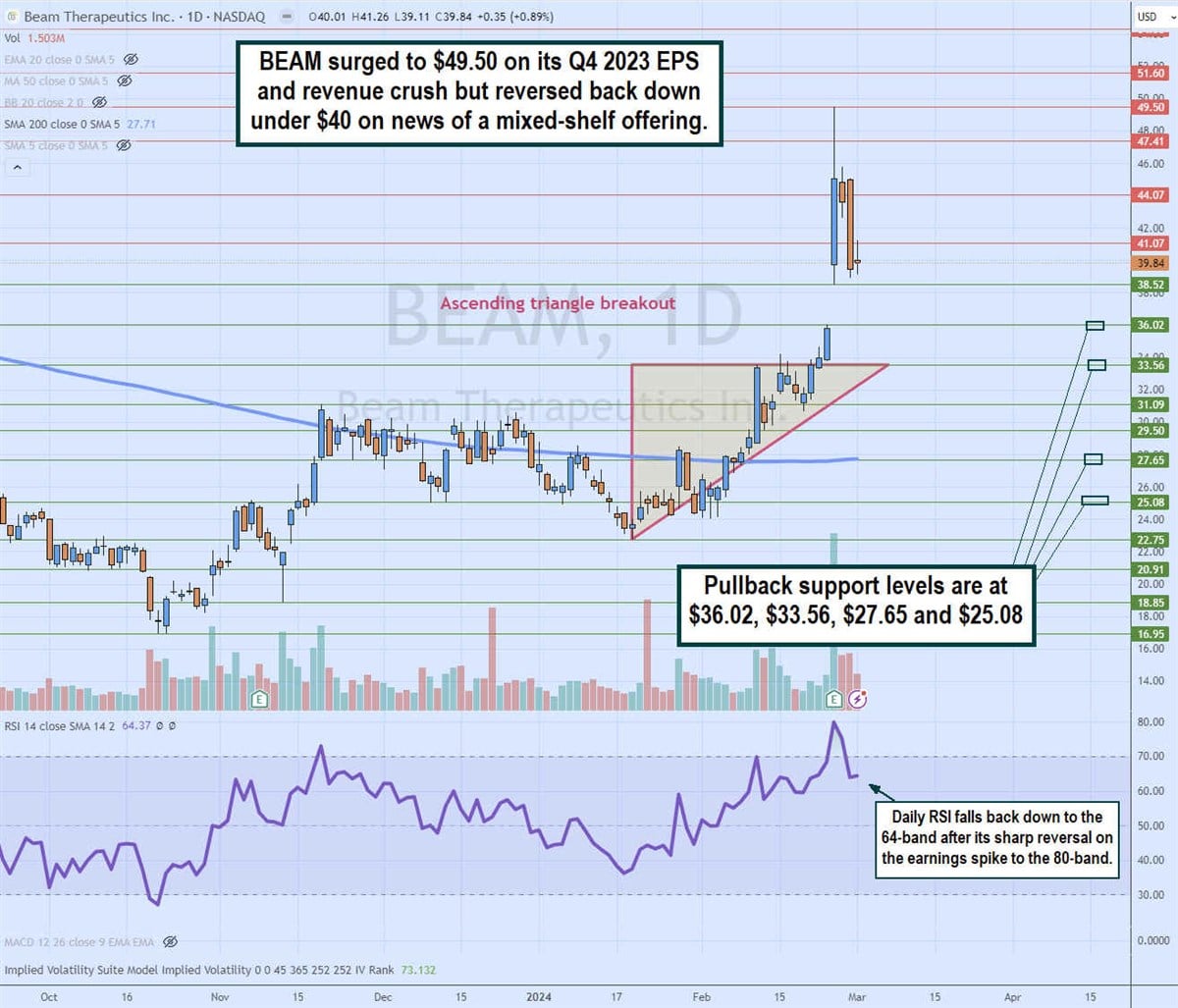
Daily breakout of the ascending triangle
The daily candlestick chart for BEAM illustrates an ascending triangle daily breakout pattern. The lower ascending trendline began at $22.75 on January 19, 2024, rising through the 200-period daily moving average (MA) at $27.65 towards flat upper trendline resistance at $33.56. The release of Q4 2023 earnings caused shares to gap to $38.52 and rally to $49.50. The daily relative strength index (RSI) rose to the 80 band and made a sharp reversal to the 64 band as the stock fell back to the $39.84 level on news of its mixed shelf offering. The pullback support levels are at $36.02, $33.56, $27.65, and $25.08.
Before you consider Beam Therapeutics, you’ll want to hear this.
MarketBeat tracks daily Wall Street’s highest-rated and best-performing research analysts and the stocks they recommend to their clients. MarketBeat identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market takes hold… and Beam Therapeutics wasn’t on the list.
While Beam Therapeutics currently has a “Hold” rating among analysts, top analysts believe these five stocks are better buys.
View the five stocks here
Wondering when you can finally invest in SpaceX, StarLink, or The Boring Company? Click the link below to find out when Elon Musk will finally allow these companies to IPO.
Get this free report