Key points
- Akebia Therapeutics is a biotech specializing in the treatment of chronic kidney disease (CKD).
- Its lead drug, Vafseo, has received FDA approval to treat anemia in adult patients with chronic kidney disease receiving at least 3 months of dialysis.
- Vafseo is an oral tablet approved in 37 countries.
- 5 stocks we like best from Akebia Therapeutics
Akebia Therapeutics Inc. NASDAQ:AKBA is a biopharmaceutical company specializing in the development of treatments for chronic kidney disease (CKD) and the management of anemia for patients with chronic kidney disease. The medical company addresses the segment’s unmet needs. It has received FDA approval for its lead candidate, Vafseo, which is used to treat anemia in adult dialysis patients with chronic kidney disease (CKD). This applies to a total addressable market (TAM) of 500,000 adult dialysis patients with anemia due to chronic kidney disease. Vafseo is an oral tablet in contrast to current treatments GSK plc NYSE:GSK using injections or blood transfusions in severe cases.
What is anemia?
Anemia is a condition triggered by the deficiency of hemoglobin in red blood cells. Hemoglobin is the protein that carries oxygen from the lungs to the body. A lack of hemoglobin causes organs to not receive enough oxygen, which can cause symptoms such as fatigue, paleness, weakness, dizziness, shortness of breath, and a fast heartbeat.
Many types of anemia, such as iron deficiency anemia and vitamin deficiency anemia, are caused by a lack of iron to make red blood cells. Hemolytic anemia forms from the premature destruction of red blood cells due to an underlying condition such as infections or autoimmune diseases. Aplastic anemia is caused by damage to the bone marrow that prevents it from producing red blood cells.
Anemia in patients with chronic renal failure
Anemia in patients with chronic kidney disease occurs when the kidneys fail to produce enough erythropoietin (EPO), a hormone that stimulates the production of red blood cells in the bone marrow. This can occur due to kidney disease, inflammatory disorders, or cancer. This results in patients having much lower red blood cells than normal, leading to anemia.
Treatment with Vafseo pill
Akebia’s main drug, Vafseo (vadadustat), treats anemia by mimicking the body’s natural response to low oxygen levels. It belongs to a class of drugs called hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors (HIF-PHI). HIF-PHIs are enzymes normally produced by healthy kidneys to help regulate HIF. Vafseo increases HIF levels by inhibiting HIF-PH enzymes. The increased levels of HIF trigger the natural production of EPO, and as more EPO is produced, the bone marrow also increases the production of red blood cells, which improves hemoglobin levels to relieve the symptoms of anemia.
Vafseo receives FDA approval
On March 28, 2024, the U.S. Food and Drug Administration (FDA) approved Vafseo tablets for the treatment of anemia with chronic kidney disease for adult patients with at least 3 months of dialysis. This overturns a previous denial of marketing approval due to safety concerns. This approval makes Vafseo approved in 37 countries as a once-daily HIF-PH inhibitor pill. This was supported by Akeba’s global Phase 3 INNO2VATE program and post-marketing safety data from Japan. Japan approved it in 2000.
The founder and president of the Renal Support Network, Lori Hartwell, has suffered from chronic kidney disease since she was a child and expressed her support: “Anemia is a debilitating condition that significantly impacts our daily lives. It is promising to see the introducing innovative therapeutic options for people battling anemia.”
Vafseo receives FDA approval
The FDA approval allows Akebia to market Vafseo in the United States with its partner CSL Limited OTCMKTS: CSLLY. CSL holds the exclusive license to sell Vafseo to third-party dialysis organizations and Fresenius Kidney Care dialysis centers. It competes with Jesduvroq, owned by GSK plc, which was approved in February 2023 and is Japan’s lead HIF-PHF drug.
Akebia CEO John Butler said, “With the approval of Vafseo in the United States, we are proud to offer an alternative treatment option for the hundreds of thousands of Americans on dialysis who have been diagnosed with anemia due to chronic kidney disease”. Butler concluded: “At Akebia we are dedicated to kidney patients, a dedication that has driven our team to reach this milestone. We believe this commitment uniquely positions the company to execute a successful launch designed to drive a potential new standard of oral care for dialysis patients.”
Results for the fourth quarter of 2023
Prior to FDA approval, Akebia released its fourth quarter 2023 earnings report on March 14, 2024. The company reported break-even earnings per share, which was 4 cents better than consensus estimates. Revenue grew 1.84% year over year to 56.2 million. The company received a $55 million term loan and $26 million in gross proceeds from at-the-market (ATM) share sales.
Akebia Therapeutics Analyst Ratings and Price Targets I’m on MarketBeat. The titles of Akebia’s competitors and competitors can be found with MarketBeat Stock Screener.
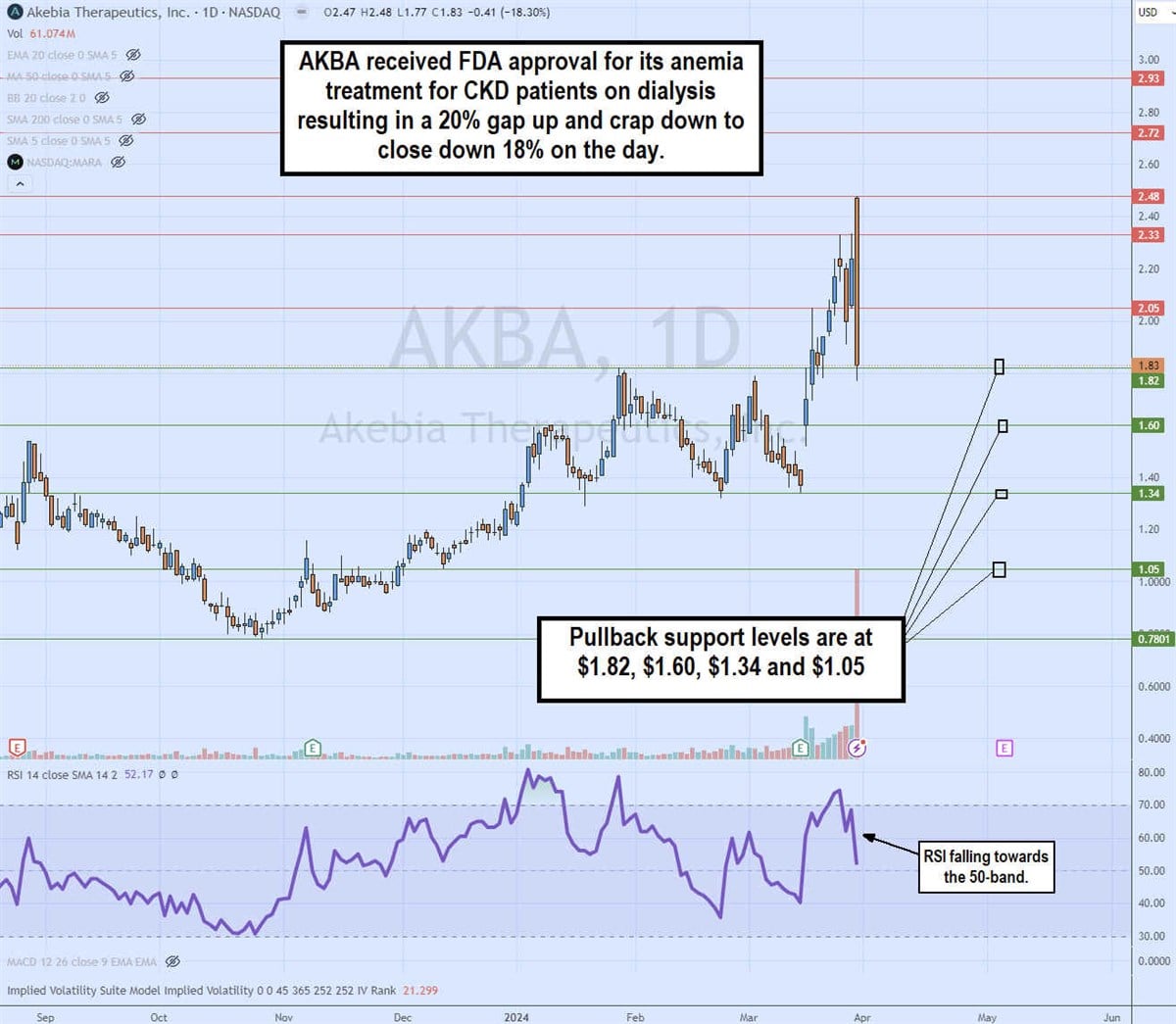
Daily Bearish Engulfing Pattern
The daily candlestick chart on PATH illustrates a bearish engulfing candlestick pattern. AKBA shares rose more than 20% on news of the FDA approval to $2.48, but formed a gap and snapback to close at $1.83, down 18% on the day . The bearish engulfing candle has engulfed the range of the previous 7 candles, which can be seen as bearish. The daily relative strength index (RSI) has fallen sharply, rejecting the 70 band to drop to the 52 band. The pullback support levels are at $1.82, $1.60, $1.34 and $ 1.05.
Before you consider Akebia Therapeutics, you’ll want to hear this.
MarketBeat tracks Wall Street’s highest-rated and best-performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market takes hold… and Akebia Therapeutics wasn’t on the list.
While Akebia Therapeutics currently has a “buy” rating among analysts, top-rated analysts believe these five stocks are better buys.
View the five stocks here
Wondering what the next big-hitting stocks with solid fundamentals will be? Click the link below to learn more about how your portfolio could blossom.
Get this free report