Key points
- In clinical trials, Wegovy patients lost an average of 16% of their body weight versus 2% of placebo users.
- Zepbound patients lost 20.9% of body weight and up to 26% after 88 weeks compared to 9.5% in the placebo group, but dosages rose to 15 mg per week compared to 2.4 mg for Wegovy.
- Both drugs are being investigated for additional indications, including cardiovascular, MASH, and neurological diseases.
- 5 stocks we like best from Novo Nordisk A/S
The GLP-1 weight loss trend continues to thrive and the stakes continue to raise. The reigning champions in the battle of the bulges are Novo Nordisk A/V NYSE: NVO with its drugs Semaglutide Ozempic and Wegovy, and Eli Lilly & Co. NYSE: LLY with its drugs Tirzepatide Mounjaro and Zepbound. Viral off-label use for weight loss has prompted both companies to rename and repurpose their diabetes drugs into obesity treatments under new, higher-dose labels called Wegovy and Zepbound.
Both medical companies are struggling to meet the insatiable demand to keep their products on shelves. Additionally, both companies are looking to expand the drugs’ indications beyond diabetes and obesity. Let’s review the two obesity treatments, Wegovy and Zepbound, to see how they stack up.
Wegs
Ozempic has made headlines thanks to social media and advertising from celebrities and influencers who have sworn by its effects on weight loss. Wegovy is a 2.4 mg weekly injection approved by the FDA specifically for obesity. During clinical trials, adults taking Wegovy lost an average of 16% of their body weight compared to 2% with the placebo. The 68-week study found that 83% of participants lost at least 5% of their weight compared to 31% of adults taking the placebo. Wegovy is approved for adults and children ages 12 and older.
Direct in Zep
Zepbound is a more potent version of Mounjaro, approved for obesity, but only in adults. Average weight loss was 20.9% after 36 weeks on open-label Zepbound. Zepbound also met secondary endpoints with improvements in BMI, fast insulin, blood pressure, lipids, and health-related quality of life, including sleep apnea. After 88 weeks, Zepbound patients lost an average of 26% of their body weight compared to 9.5% in the placebo group. The percentage of patients who lost at least 20% of their body weight was 72.6% for Zepbound users compared to 11.6% for the placebo.
However, it is important to note that in the Surmount-4 study, patients were given 2.5 mg of Zepbound and continued to increase the dosage to maximum tolerated levels of 10 mg or 15 mg once a week. The starting dose was 2.5 mg and was increased by 2.5 mg every 4 weeks until the maximum dosage of 10 mg or 15 mg was reached.
Differences in mechanisms
While both drugs are approved treatments for obesity that mimic the hormone GLP-1, the gut hormone that regulates blood sugar levels and appetite, leading to reduced calorie intake, Zepbound is a dual agonist. It also mimics another cleaved hormone called glucose-dependent insulinotropic polypeptide (GIP), which works together with GLP-1 to generate a stronger cumulative effect. This has been proven to show greater weight loss.
Differences in dosage
Just like in clinical trials, the dosages are also different. It is suggested to start with Wegovy at 0.25 mg for 4 weeks. Patients then gradually increase the dosage to 0.5 mg, then 1 mg, then 1.7 mg up to 2.4 mg at the highest dosage.
Zepbound administration begins with 2.5 mg for the first 4 weeks, then increases to 5 mg. The dosage may be increased up to 10 mg or 15 mg based on tolerability.
Differences in costs
Health insurance coverage varies by carrier. While many insurers cover GLP-1 drugs for type 2 diabetes, they may not cover obesity. Both drugs are once-weekly injections. Wegovy tends to be more expensive than Zepbound. GoodRx Health NASDAQ: GDRX has a list price on Wegovy of $1,590.29 for a 4-week box discounted to $1,388.64. It has Zepbound listed at $1,250.08 for a 4-week supply carton discounted to $1,095.66.
Differences in indications
Wegovy was approved for cardiovascular risk reduction in March 2024. Over 5 years, Wegovy was found to reduce the risk of a major adverse cardiovascular event (MACE) by 20%. It has also been shown to improve inflammation and distance walked.
Zepbound is effective for fatty liver disease. In a Phase 2 study, 74% of patients taking Zepbound achieved freedom from non-alcoholic steatohepatitis (NASH) without worsening of liver fibrosis compared to 12.6% in the placebo group. GLP-1 is being studied to stop the worsening of Parkinson’s disease.
On paper, it appears that Zepbound may be the superior weight loss drug offered at a lower price than Wegovy, acting as a dual agonist that mimics 2 gut hormones instead of just GLP-1. Lilly shares indicate a pullback is underway. However, while Novo Nordisk and Ely Lilly are kings of the hills for now, there are several GLP-1 candidates in the works, including VK2735 from Viking Therapeutics Inc. NASDAQ: VKTX and Pemvidutide from Altimmune Inc. NASDAQ:ALT.
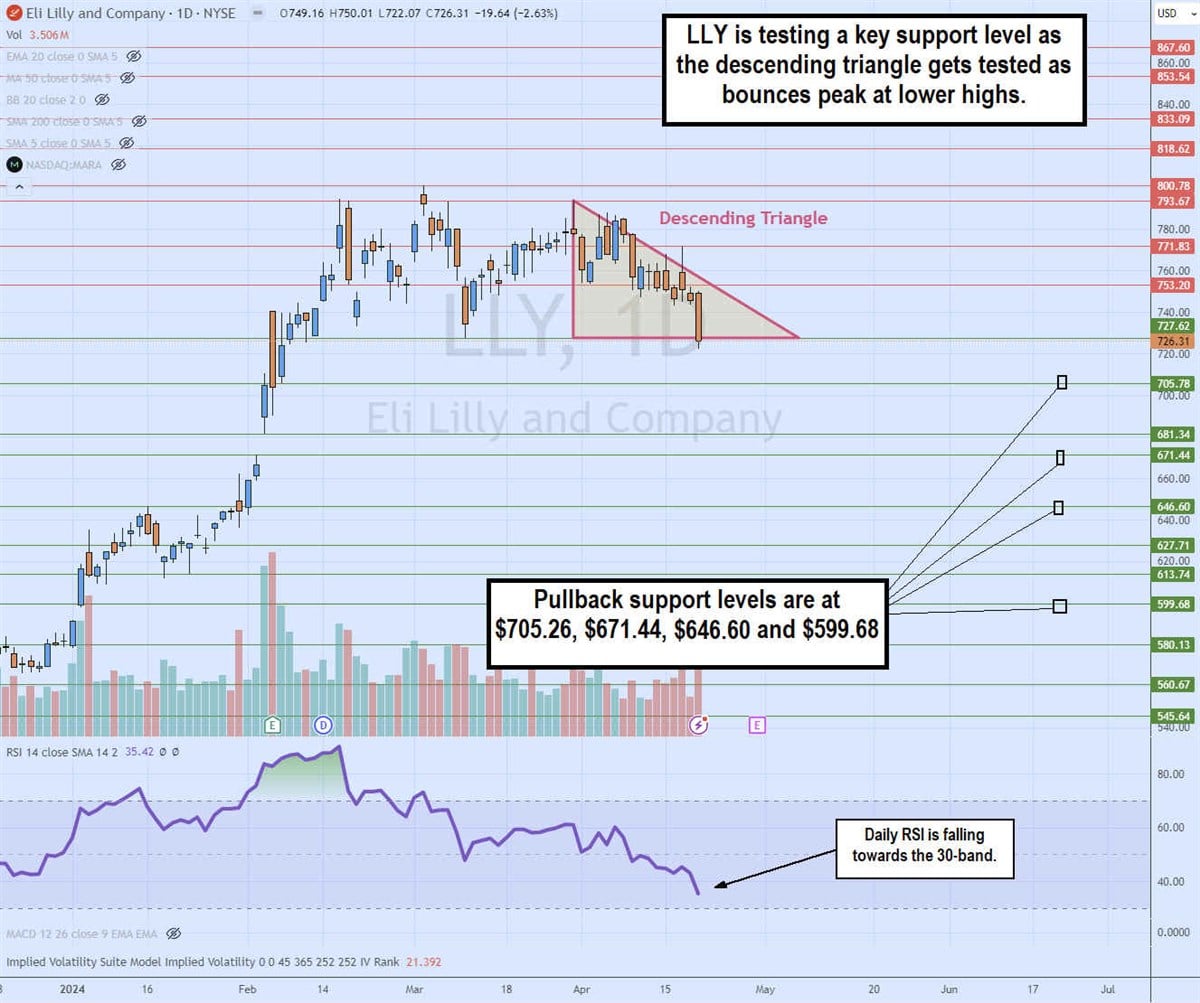
The daily candlestick chart on LLY indicates a descending triangle pattern. The descending trendline formed at $793.67 on March 24, 2025, limiting bounces to lower highs. The flat-bottomed trendline formed at $726.31. The daily relative strength index (RSI) continues to decline towards the 30 band. The pullback support levels are located at $705.26, $671.44, $646.60, and $599.68.
Before you consider Novo Nordisk A/S, you’ll want to hear this.
MarketBeat tracks Wall Street’s highest-rated and best-performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat has identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market takes hold… and Novo Nordisk A/S wasn’t on the list.
While Novo Nordisk A/S currently has a “Moderate Buy” rating among analysts, top analysts believe these five stocks are better buys.
View the five stocks here
MarketBeat just released its list of 10 cheap stocks that have been overlooked by the market and may be seriously undervalued. Click the link below to see which companies are on the list.
Get this free report