Key points
- CRISPR Therapeutics made global headlines in 2024, receiving the first FDA approval for a gene-editing therapy for sickle cell disease (SCD) and transfusion-dependent beta-thalassemia (TDT).
- Next-generation gene editing will evolve Cas9 from molecular scissors to a molecular UPS truck that delivers cargo to a specific part of the genome.
- CTX-211 is in Phase 1 clinical trials for the treatment of type 1 diabetes (T1D) with the goal of allowing patients to produce their own insulin instead of relying on daily insulin injections, as 10% of all diabetes cases worldwide are T1D.
- 5 stocks we like better than CRISPR Therapeutics
CRISPR Therapeutics AG NASDAQ: CRSP made headlines around the world when he became partner Vertex Pharmaceuticals Inc. NASDAQ: VRTX received the first FDA approval for a gene-editing therapy for sickle cell disease (SCD) called Casgevy on December 8, 2023. This FDA approval was a boon for the medical industry and especially for therapy-focused biotechs gene like Intellia Therapeutics Inc. NASDAQ: EXCLUSIVE AND Fascio Therapeutics Inc. NASDAQ: BEAM. bluebird organic Inc. NASDAQ: BLUE also simultaneously received FDA approval for its gene therapy treatment for sudden heart disease called Lyfgenia on December 8, 2023.
Similar impact to Nvidia
The impact on profits has been dramatic, paralleling the impact of the artificial intelligence (AI) boom. Nvidia Co. NASDAQ:NVDA final results. Just as artificial intelligence went mainstream and viral in 2023, gene therapy and gene editing could reach their tipping point in 2024. Casgevy has now received approval in the European Union, Britain, Saudi Arabia and Bahrain, with more nations to come. Proposed legislation for the treatment of SCD and TDT is under review in Switzerland and expected for Canada in the first half of 2024. Check the heat map of the sector on MarketBeat
Similar results to Nvidia
On February 21, 2024, CRISPR Therapeutics announced its fourth-quarter 2023 earnings results. The company reported EPS of $1.10, beating consensus analyst estimates of 15 cents by 95 cents, or 7 times. Revenue increased 3,235% to $201.2 million versus $148.72 million. Revenue comes from major payments, licensing and royalties.
Casgevy is a single treatment that costs $2.2 million per patient. Vertex has activated 12 licensed treatment centers across the United States and has signed with Synergie Medication Collective (SMC) to provide access to Casgevy. Obtain AI-powered insights on MarketBeat.
SMC is a contracting organization that supports payers covering nearly 100 million people in the United States. Vertex has been corresponding with the state’s Medicaid organization to secure immediate coverage and reimbursement for Casgevy. The company has $2.1 billion in Pro-Forma cash and cash equivalents as of February 21, 2024.
Candidates for next-generation immuno-oncology and autoimmune diseases
CRISPR Therapeutics has ongoing clinical trials for CAR T product candidates (CTX112 targeting CD19 and CTX131 targeting CD70). Pharmacological data indicate that novel potency modifications in CTX112 and CTX131 can lead to much higher CAR T cell expansion compared to first-generation candidates. Clinical updates for the next-generation candidates will be released later in the year.
Samarth Kulkarni, CEO of CRISPR Therapeutics, commented: “So the picture of a Cas9, CRISPR/Cas9, goes from molecular scissors to a sort of molecular UPS truck that delivers cargo to a particular part of the genome where a protein loads effector to perform an operation”. desired modification on the genome and whether it is a reverse transcriptase or a MNase.”
Clinical study on the treatment of type 1 diabetes (T1D).
CRISPR Therapeutics is in Phase 1 clinical trials for CTX211 for the treatment of type 1 diabetes (T1D), also known as insulin-dependent diabetes. CTX211 is an investigational allogeneic, genetically modified, stem cell-derived therapy for the treatment of T1D. It aims to incorporate genetic modifications to make it hyperimmune and improve the fitness of cells. This therapy is designed for allow patients to produce their own insulin in response to glucose.
Information about type 1 diabetes
T1D usually requires vital insulin therapy administered via daily injections. TD1 is usually diagnosed in childhood and adolescence, but it can occur at any age and is widely believed to be caused by genetic factors. Unlike type 2 diabetes, T1D cannot be prevented by lifestyle changes. There are approximately 1.6 million people in the United States with T1D, and nearly 10% of all diabetes cases are affected by T1D globally.
Vertex paid CRISPR Therapeutics $170 million in upfront and milestone payments for non-exclusive rights to treat T1D with an additional $160 million in research and development milestones along with royalties on any future products.
Comments from CTX211 at the Citi Virtual Oncology Leadership Summit
Samarth Kulkarni, CEO of CRISPR Therapeutics, addressed CTX211 and the changes made to some genes such as HLAE and PD-L1 in addition to the beta 2M modification. The main goal is to understand how the modified cells interact with the immune system. The goal is to ensure that these cells avoid being attacked by the body’s immune system and, therefore, have to be masked or made invisible. If they can avoid attack, the modified cells have the potential to allow insulin production without being destroyed by the immune system.
Kulkarni summed it up like this: “I think the fundamental question here is: can we get hyperimmune or hypoimmune cells that are invisible and protect themselves from the host immune system? And if this is something that we achieve with these cells, everything else is titratable. We can increase the cell dose for greater insulin production, or we can reduce it if necessary. But the fundamental question is: are these cells capable of evading the immune system?”
CRISPR Therapeutics Analyst Ratings and Price Targets I’m on MarketBeat. CRISPR Therapeutics peers and competing stocks can be found with MarketBeat Stock Screener. CRSP stock has a short interest of 20.06%.
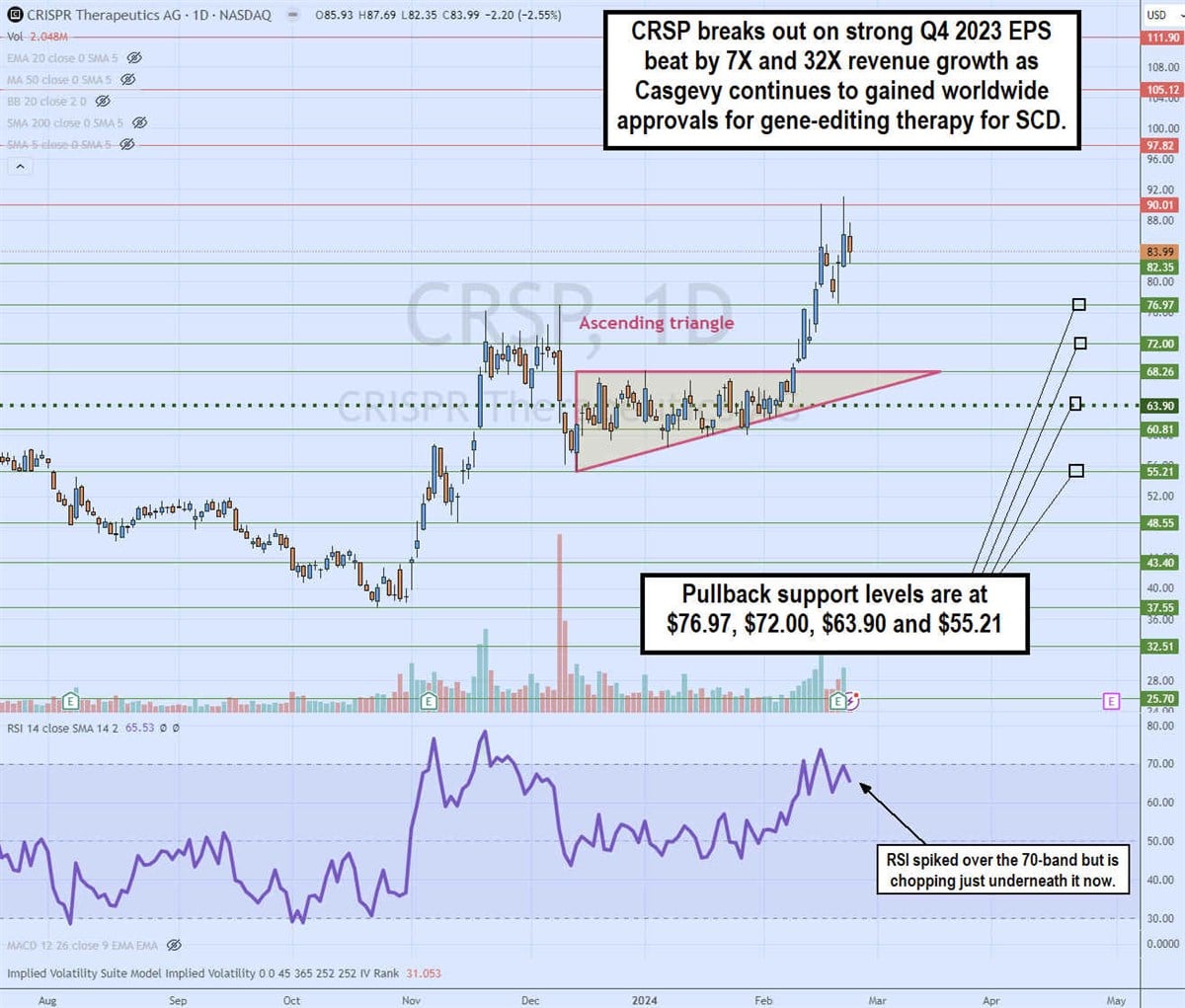
Ascending Triangle Daily Breakout
The daily candlestick chart on CRSP illustrates an ascending triangle breakout pattern. The ascending trendline formed at $55.21 on December 13, 2023, rising towards flat horizontal trendline resistance at $68.26. The daily market structure low (MSL) was triggered at $63.90.
The breakout formed on February 8, 2024, sending shares as high as $91.18 on the release of fourth-quarter 2023 earnings. The relative strength index (RSI) surged above 70 bands, and then reduce. The pullback support levels are at $76.97, $72.00, $63.90, and $55.21.
Before you consider CRISPR Therapeutics, you’ll want to hear this.
MarketBeat tracks Wall Street’s highest-rated and best-performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market takes hold… and CRISPR Therapeutics wasn’t on the list.
While CRISPR Therapeutics currently has a “Hold” rating among analysts, top-rated analysts believe these five stocks are better buys.
View the five stocks here
MarketBeat analysts just released their top five short stocks for March 2024. Find out which stocks have the most short interest and how to trade them. Click the link below to see which companies are on the list.
Get this free report