
Key points
- Viking Therapeutics is a clinical-stage biotech specializing in treatments for metabolic and endocrine disorders.
- Its lead drug candidate, VK2735, a GLP-1/GIP dual agonist, showed promising weight loss during Phase I and Phase 2 trials, with minimal to mild side effects.
- Its oral pill version has completed its Phase 1 clinical trial with solid results and is poised to move into Phase 2, as Oppenheimer raised its price target for VKTX to $138 per share.
- 5 stocks we like better than Viking Therapeutics
GLP-1 (Semaglutide) drugs such as Ozempic and Wegovy produced by Novo Nordisk A/S NYSE: NVO and Mounjaro and Zepbound (Tirzepatide) produced by Eli Lilly and Co. NYSE: LLY have taken the mainstream by storm. These drugs have transformed the medical industry. Oprah Winfrey’s special, “Shame, Guilt, and the Weight Loss Revolution,” has drawn more attention to the benefits of clinical weight loss treatments since she calls obesity a disease. Obesity can increase the risk of over 200 diseases. The popularity of GLP-1 drugs has caused a gold rush for biotechs looking to profit from the growing demand that affects nearly 60% of the world’s population.
Viking Therapeutics Inc. NASDAQ: VKTX has one of the most promising drugs yet to hit the market. The company specializes in the treatment of metabolic and endocrine disorders. Viking’s lead drug candidate is VK2735. Like Mounjaro, VK2735 is a dual agonist for both the GLP-1 hormone and glucose-dependent insulinotropic polypeptide (GIP). Its clinical trials are going so well that many brokers have come out ahead of FDA approval requests and given stock price targets of over $100. Oppenheimer raised his price target to $138. Viking has a pipeline of drugs that also address obesity-related diseases, such as VK2809, a treatment for nonalcoholic steatohepatitis (NASH) and fatty liver. It attempts to reduce fat in the liver to prevent it from causing inflammation and damage, leading to cirrhosis and potentially death.
The miscategorization of body mass index
Body mass index (BMI) is used to define overweight people above the age of 25, while obese people are defined by a BMI over 30. Nearly 60% of the population falls into the overweight to obese category. However, critics argue that BMI only measures weight and height without considering overall body composition and shape. The BMI would have classified Arnold Schwarzenegger and all heavyweight bodybuilders as obese individuals.
The triple threat
Current GLP-1 drugs are once-weekly injectable treatments with side effects ranging from nausea, vomiting and diarrhea to gastrointestinal pain. Mounjaro saw more clinical trial participants drop out due to gastrointestinal side effects than Ozempic. Most users stop taking GLP-1 drugs after a year due to painful side effects.
Viking therapy has shown greater tolerability to side effects, which may not be as serious. Its clinical studies have also shown comparable or even greater weight loss than Ozempic and Zepbound.
Phase 1 injectable clinical trials of VK2735
The Phase I clinical trials of VK2735 were successful and met safety and tolerability endpoints, as no patients dropped out of the two studies due to adverse side effects. This is a significant advantage that neither Ozempic nor Mounjaro can claim. Clinical studies of VK2735 injectable were conducted using a single ascending dose (SAD) and a multiple ascending dose (MAD) study for obese patients with a BMI of 30 or greater. The mean reduction in body weight from baseline was was up to 7.8% after 21 days. All treatment-emergent adverse events were tolerable with mild to moderate severity, with no serious adverse events.
Phase 2 trials
The Phase 2 VENTURE studies included 176 adult participants with increased doses. The primary and secondary endpoints were all successfully achieved. The treatments were safe and well tolerated, with most side effects classified as mild and moderate. Patients lost up to 14.7% of baseline body weight after 13 weeks, with significant differences between the placebo group. At least 88% of participants lost at least 10% weight compared to 4% in the placebo group.
Regarding safety and tolerability, 13% discontinued the study compared to 14% of the placebo group, and 92% of participants reported side effects of mild or moderate severity. Gastrointestinal tract-related adverse effects were seen early in the study and decreased with frequency. The weekly rate of nausea did not exceed 5% at any time after the first week. Viking plans to meet with the FDA to discuss next steps toward seeking approval. Check the heat map of the sector on MarketBeat.
Positive results from the oral tablet phase 1 study
An oral tabletop version of VK2735 has completed Phase 1 clinical trials with positive results. The 28-day MAD study demonstrated an average reduction in body weight from baseline of up to 5.3 and compared to placebo at 3.3%. Up to 57% of participants achieved weight loss of at least 5% compared to 0% on placebo. A treatment duration longer than 28 days would likely provide greater weight loss, which will be accomplished in Phase 2.
Bian Lin, Ph.D., CEO of Viking Therapeutics, commented: “We are particularly pleased with the initial safety and tolerability data, which suggest a differentiated profile with minimal GI-related side effects. We believe that an oral agent with good tolerability could represent an interesting potential treatment option for patients with obesity. We look forward to seeing a longer treatment window and potentially higher dose in an upcoming Phase 2 study.”
Viking Therapeutics analyst ratings and price targets I’m on MarketBeat. You can find competing and competing stocks of Viking Therapeutics with MarketBeat Stock Screener.
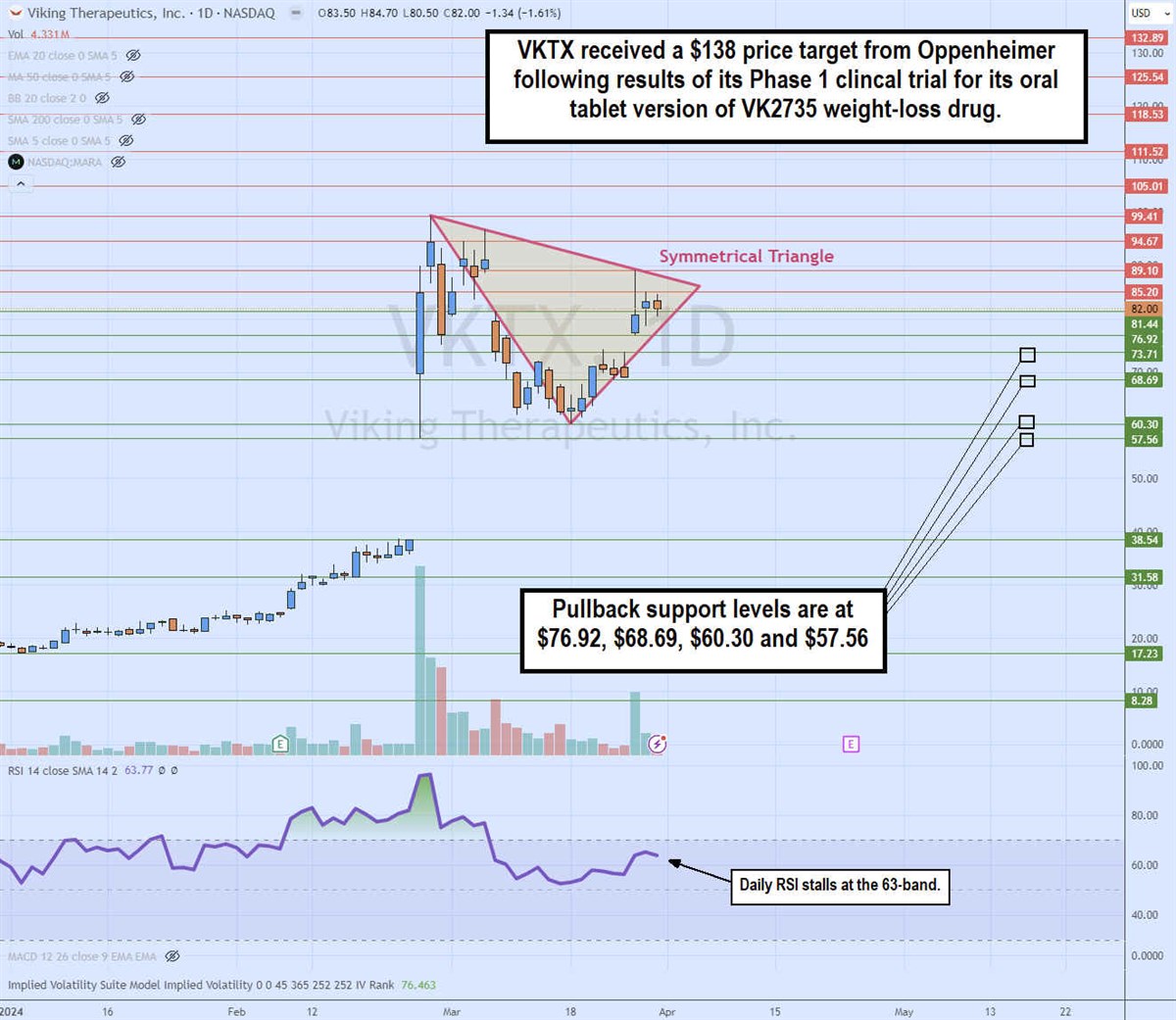
Daily symmetrical triangle
The daily candlestick chart for VKTX illustrates a symmetrical triangle daily pattern. It could also be considered a pennant as the flag pole is evident on the blank. The descending trend line formed from the high of $99.41 on February 28, 2024. The ascending lower trend line formed at the low of $60.34 on March 18, 2024. The trend lines converge at the apex point in where a breakout through the descending trend line will trigger a breakout, or a break through the ascending trend line will trigger a breakout. The daily relative strength index (RSI) is stalled at the 63 band, ready to bounce above the 70 band or retreat. The pullback support levels are at $76.92, $68.69, $60.30, and $57.56.
Before you consider Viking Therapeutics, you’ll want to hear this.
MarketBeat tracks Wall Street’s highest-rated and best-performing research analysts and the stocks they recommend to their clients on a daily basis. MarketBeat identified the five stocks that top analysts are quietly whispering to their clients to buy now before the broader market takes hold… and Viking Therapeutics wasn’t on the list.
While Viking Therapeutics currently has a “buy” rating among analysts, top-rated analysts believe these five stocks are better buys.
View the five stocks here
MarketBeat analysts just released their top five short stocks for April 2024. Find out which stocks have the most short interest and how to trade them. Click the link below to see which companies are on the list.
Get this free report